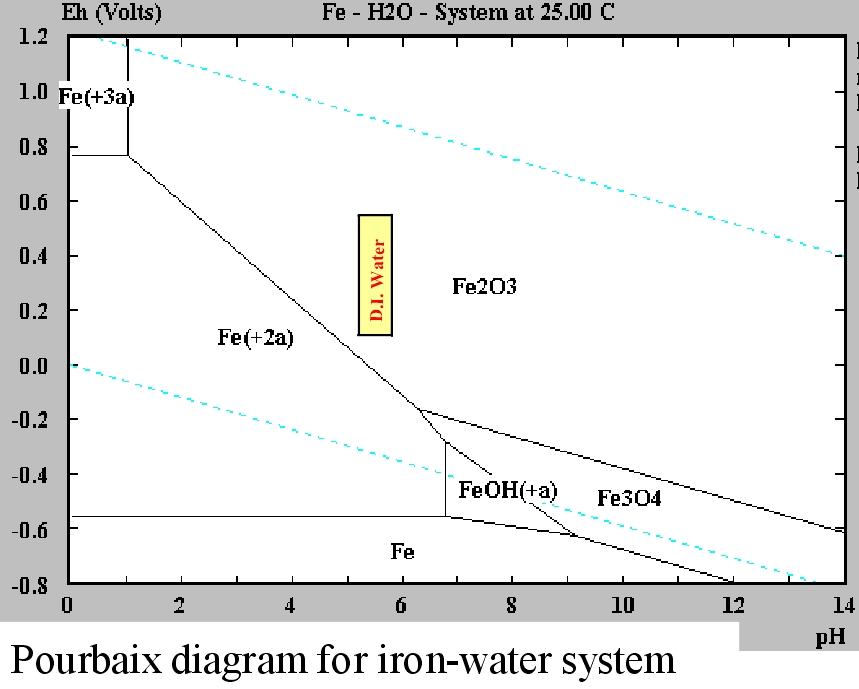
Iron Pourbaix Diagram
 |
Corrosion Potentials:
The Pourbaix diagram indicates that iron forms an oxide in D.I. water. However, iron oxide has been extensively studied and this oxide does not form a continuous or passive coating. The formation of iron oxide, especially at higher pH values actually results in increased pitting corrosion.
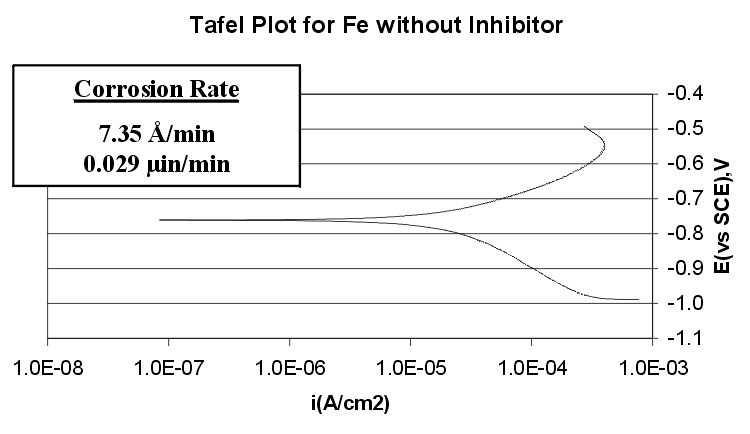
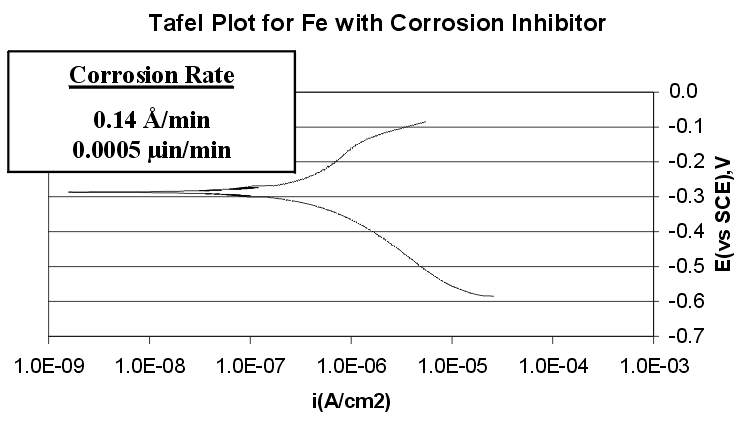
Corrosion Rates:
The Tafel plots shows that with the addition of a corrosion inhibitor, the corrosion rate of iron can be reduced from 7.35 angstroms/minute to 0.14 Angstroms/minute.
 |
 |
